Liu Lab
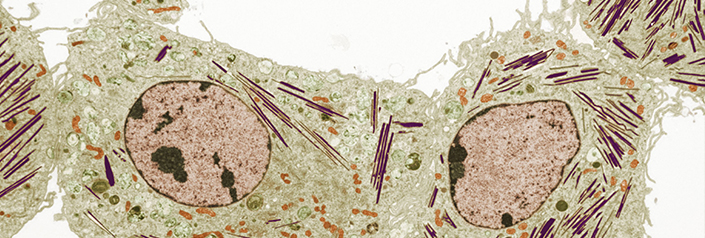
In the Liu Lab at NYU Langone, our current research interests include 1) musculoskeletal development and disorders, with particular focuses on osteoarthritis and fracture healing; 2) inflammation and autoimmunity, with a special focus on rheumatoid arthritis; and 3) rare genetic lysosomal storage diseases, including Gaucher disease and Tay-Sachs disease.
Contact Us
Chuanju Liu, PhD
Principal Investigator
Professor, Departments of Orthopedic Surgery and Cell Biology
301 East 17th Street
16th Floor
New York, NY 10003
Phone: 212-598-2373
Fax: 212-598-6096
Email: chuanju.liu@nyulangone.org
