
Rifkin Lab Research
Research in the Rifkin Lab is focused on elucidating how transforming growth factor-β (TGF-β) is regulated. The TGF-βs (β1, β2, β3) are 25kD dimeric cytokines derived from larger precursors by intracellular processing by furins. However, even though the bonds between the TGF-β dimer and the propeptide dimer are cleaved within the trans Golgi, the propeptide remains tightly associated with the growth factor by non-covalent interactions. This association renders the TGF-β unable to interact with its receptor. Thus, TGF-β is normally secreted in a latent form and to act must be liberated from interaction with its propeptide, also known as the latency associated protein (LAP).
The extracellular forms of latent TGF-β are also associated with a second gene product, the latent TGF-β binding protein (LTBP). LTBPs are complex proteins consisting of multiple EGF-like domains and signature 8-cysteine-containing modules. LTBPs are joined to LAP via a pair of disulfide bonds that form between cysteine residues near the amino terminus of LAP and a pair of cysteines in the third 8-cysteine domains of LTBP-1, 3, or 4.
Our research efforts are focused on several themes that relate to the fundamental biology of TGF-β and its activation. First, we are interested in elucidating the mechanisms that convert latent to active TGF-β. We have described activation by proteases and by the integrin alpha vβ6. We are currently performing molecular experiments to establish the role of LTBP in this process, the regions of LTBP that are involved, and the biochemical constituents that are part of the activation reaction. We have also developed a genetic screen for molecules that activate latent TGF-β. We have isolated several cell clones that may express novel activators and are in the process of characterizing these activators. Our ultimate goal is to understand latent TGF-β activation in a way that will permit the design of inhibitors for specific activation pathways.
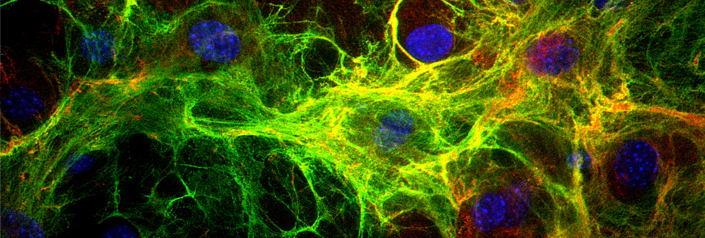
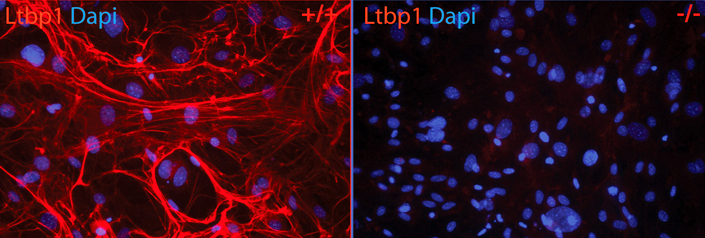
Second, we are studying LTBP function by creating mouse mutants in which either null mutations have been introduced into the LTBP genes, mutations have been made that affect LTBP function, or point mutations in LAP have been generated that will block binding to LTBP. These mutations have revealed several interesting phenotypes in bone, lung, and fat differentiation are informative with respect to aspects of TGF-β biology.
Finally, we are interested in the role of the LTBPs during early development. We have found that these proteins may participate in patterning of the dorsal ventral axes in early embryos. We think that this occurs via interactions with the TGF-β superfamily members activin and nodal. We are interested in understanding the developmental consequences of these interactions as well as the biochemical parameters that control them.