Kirsch Lab
Kirsch Lab Research

Kirsch Lab Research
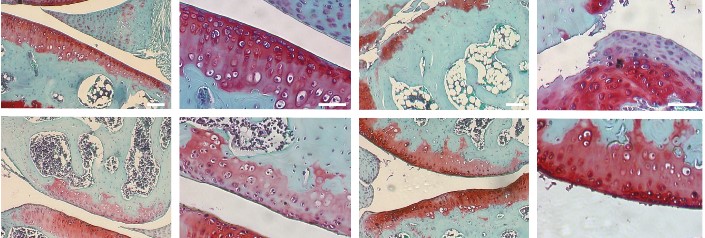
Research in the Kirsch Lab focuses on three main areas:
- the role of annexins in chondrocyte differentiation events during development and pathology
- the role of the progressive ankylosis gene (ank) in physiological and pathological mineralization
- the role of the progressive ankylosis gene (ank) in mesenchymal stem cell commitment toward a specific cell lineage
Funding
Projects led by Thorsten Kirsch, PhD, have been funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, and the Arthritis Foundation.