
The Role of the Progressive Ankylosis Gene in Mesenchymal Stem Cell Commitment Toward a Specific Cell Lineage
Embryonic and adult stem cells are multipotential and can differentiate into multiple cell lineages. Skeletal tissue cells are derived from mesenchymal stem cells, which can differentiate along several lineages, including fibroblasts, muscle cells (myoblasts), fat cells (adipocytes), cartilage cells (chondrocytes), and bone cells (osteoblasts). To use stem cells for tissue-engineered repair, it is important to understand the mechanisms involved in them along the path to a particular cell lineage.
Investigators in the Kirsch Lab have found evidence that the progressive ankylsosis gene (ank) is required for the commitment of mesenchymal stem cells along the osteoblast lineage. We have shown that loss of Ank function results in delayed differentiation of preosteoblastic cells toward immature osteoblasts and a differentiation of these preosteoblastic cells along the chondrocytic lineage. Loss of Ank function in these preosteoblastic cells was found to result in a marked decrease the expression of osterix—a key transcription factor required for the differentiation of preosteoblastic cells into immature osteoblasts. Loss of osterix results in a total loss of bone formation. Therefore, Ank appears to be upstream of osterix and regulates its expression and ultimately the differentiation of precursor cells along the osteoblastic lineage.
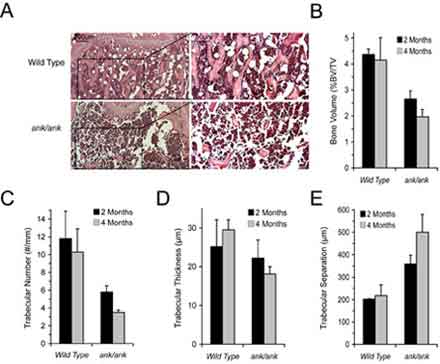
Hematoxylin and eosin staining of sections from the proximal tibiae of four-month-old ank/ank mice (ank/ank; a, b) and wild-type littermates (WT; c, d). Bar = 200 µm. Panels b and d show higher magnification of the area outlined in panels a and c. Bar = 50 µm.(B-E) Quantitative histomorphometry showing significantly lower bone volume/total volume, trabecular number, and increased trabecular separation in two- and four-month-old ank/ank mice compared to wild-type littermates. Trabecular thickness was significantly decreased in four-month-old ank/ank mice but not in two-month-old ank/ank mice (*p < .01 vs. wild-type littermates; n = 5 per genotype group).
Our current studies focus on how Ank regulates precursor cell commitment toward a specific cell lineage. We have found that transgenic mice that have a complete loss of Ank function exhibit an osteopenic phenotype in long bones as well as severe pathologic mineralization in joints, intervertebral discs, and aortas; these animals die by the fourth month of age.
Interestingly, heterozygous mutations of ank in humans result in a similar, slightly milder version of the phenotype in homozygous mice. These studies, which promise to enhance our understanding of the mechanisms regulating early osteoblastogenesis, suggest that Ank regulates bone formation not only during development but also during bone remodeling and therefore may be a novel candidate gene important in the development of osteoporosis, a disease that is characterized by loss of bone mass and strength and that often results in bone fractures from even minor trauma. Understanding the factors and mechanisms regulating cell lineage commitment is moreover crucial for the use of stem cells in tissue repair.