
The Role of Annexins in Chondrocyte Differentiation Events During Development & Pathology
During endochondral ossification, long bones are first cartilaginous. During this process, chondrocytes in the growth plate undergo a series of differentiation events, including proliferation, hypertrophy, terminal differentiation, mineralization, and programmed cell death (apoptosis). Eventually, apoptotic chondrocytes and mineralized cartilage are replaced by bone.
Once the long bones have reached their final size, most of the cartilage is replaced by bone except for a small rim of cartilage—the articular cartilage—that plays a crucial role in normal joint function. Destruction of articular cartilage is associated with osteoarthritis and rheumatoid arthritis.
The Kirsch Lab is interested in how terminal differentiation, mineralization, and apoptotic events in chondrocytes are regulated during normal development and under pathological conditions such as osteoarthritis. We have shown that retinoic acid (vitamin A) regulates terminal differentiation, mineralization, and apoptotic events in growth plate chondrocytes. Treatment of growth plate chondrocytes with retinoic acid leads to calcium channel formation of annexins II, V, and VI. Annexins are cytoplasmic proteins, which in the presence of calcium bind to acidic phospholipids.
Our studies have characterized the mechanisms leading to membrane binding and channel formation of annexins II, V, and VI and the consequences of alterations of calcium homeostasis mediated by these annexins in chondrocyte differentiation. These studies have demonstrated that blocking annexin channels inhibits terminal differentiation events of growth plate chondrocytes. Interestingly, these annexins bind to extracellular matrix molecules and cytoskeletal proteins. Therefore, these studies test the hypothesis that annexins act as mechanosensitive calcium channels controlling chondrocyte differentiation.
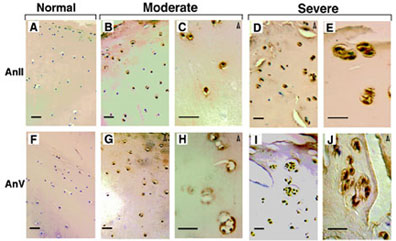
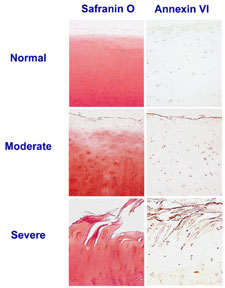
Our laboratory was the first to show that articular chondrocytes in osteoarthritic cartilage undergo similar terminal differentiation events as growth plate chondrocytes. Whereas these events are crucial for endochondral bone formation, they lead to cartilage destruction when occurring in articular chondrocytes. Annexins II, V, and VI are highly expressed by articular chondrocytes in osteoarthritic cartilage, but not in healthy articular cartilage. In addition, we detected other terminal differentiation markers in osteoarthritic cartilage.
Therefore, we hypothesize that articular chondrocytes lose their phenotype in osteoarthritis and undergo similar differentiation events as growth plate chondrocytes, leading to terminal differentiation, mineralization, and apoptosis. Furthermore, we propose that annexins are crucial regulators of these differentiation events during development and pathology and that interfering with annexin functions may provide novel therapeutic targets to stop or slow down the progression of osteoarthritis.