
Basu Lab Research Methods
Investigators in the Basu Lab capitalize on the ability to electrically and optically monitor activity in acute brain slices and in brains of live animals during behavior. Optogenetically manipulating selective hippocampal and cortical inputs during dendritic recordings helps us capture how long-range projection circuits mediate dendritic spikes and plasticity in brain slices.

Our lab is one of a handful that performs long-term intracellular recordings in mice from submicron thin dendritic processes, the loci of input integration. In parallel, two-photon imaging of cortical axons, dendrites, and cell bodies in head-fixed mice during contextual learning provides a high-resolution measure of behaviorally relevant population dynamics. This provides us an integrated view of circuit function in shaping single neuron computation and behaviorally relevant output.
Electrophysiology
We use somatic and dendritic patch clamp electrophysiology in acute brain slices to gain access into compartmentalized intracellular dynamics of the neuron. We measure intrinsic and extrinsic properties of various cell types to examine synaptic physiology and functional connectivity. Our experimental designs especially cater to looking at spatio-temporal integration of inputs, coincidence detection and plasticity.
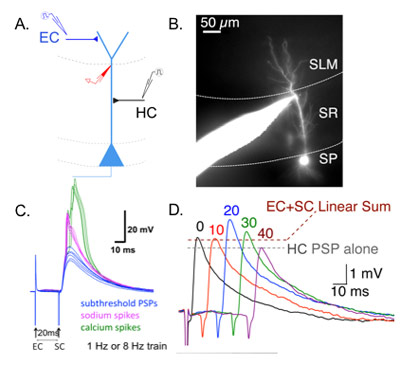
Imaging
Two-photon functional imaging in brain slices and in awake behaving mice is a technical driving force for several of our projects. This method provides high spatial and temporal resolution in monitoring activity in genetically defined neuronal populations and their subcellular compartments.
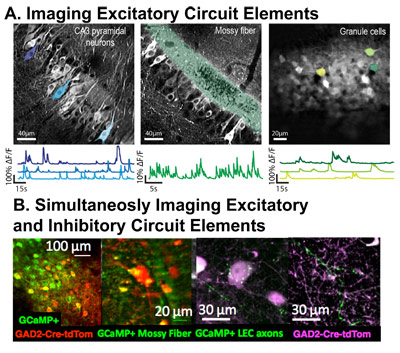
Single Molecule Imaging
We have also delved into the world of inter-molecular and intra-molecular interactions within synaptic boutons with live cell fluorescent imaging techniques such as FRET, FRAP and FLIM.
Behavior
Our behavioral testing comes in two flavors: classical freely moving behavioral paradigms such as operant fear conditioning, spatial working, and reference memory; and head-fixed behavioral tests in a virtual reality environment. We like mixing these tasks with electrophysiology and imaging.
Molecular and Genetic Manipulations of Neurons
Our combinatorial genetics strategies rely on cell-type specific recombinase expressing transgenic driver mouse lines and stereotaxic delivery of recombinant viruses to selectively target and express the following genetically encoded proteins:
- optogenetics: light gated ion channels and pumps for functional manipulations
- fluorescent reporter molecules and genetically encoded Ca2+ indicators for optical tracking
- pharmacogenetics: ligand gated ion channels for functional manipulations
- anatomical mapping with monosynaptic retrograde labeling with pseudo-typed rabies
- intersectional and promoter specific genetic targeting