
Research
Extracellular matrix in the healthy cornea
A major focus in our laboratory is understanding cell-ECM interactions and their impact on the structure and functions of the cornea. The transparent cornea provides two-thirds of the refractive power of the eye and serves as a protective barrier through unique adaptations of the cells and the ECM. The stromal ECM connective tissue is a highly organized array of collagen fibrils and regulatory proteoglycans produced by a specialized type of fibroblast, the keratocytes (Scott, 2011). We are investigating properties of keratocytes, their development, and whether these cells can be maintained in culture to produce a cornea-like stromal ECM (Foster, 2018). The stromal ECM has precisely stacked, uniformly thin collagen fibrils that gives the cornea its transparent and refractive qualities for optimal vision. We discovered that lumican is a key proteoglycan that helps to assemble uniform collagen fibrils, and gene-targeted lumican-null mice have thick, laterally fused collagen fibrils and develop corneal opacity (Chakravarti, 1998; Chakravarti, 2006).
We are developing cornea organoids using induced pluripotent stem cells to explore cell-ECM interactions in a 3D cell culture model system (Foster, 2017). These organoids mimic certain features of the cornea and therefore may be used to understand early events in the development of the cornea. The organoids are also being used in functional analyses of genes active in the cornea.
Pathogenesis and genetic causes of keratoconus
Keratoconus, a degenerative thinning and weakening of the corneal ECM, leads to bulging of the cornea with progressive loss of vision. Affecting 1 in 1000 people, it is the second-most-common reason for cornea transplants worldwide. While the etiology of keratoconus is not fully understood, interactions of genetic factors with environmental stressors are suspected. Our ongoing work on keratoconus is investigating epithelial and keratocyte dysfunctions, and the altered ECMs they produce. The pathogenic cellular and ECM changes in keratoconus are increasingly thought to be due to the accumulation of multiple deleterious genetic variants in an individual. Our collaborative and interdisciplinary team is conducting whole exome and genome sequencing to identify pathogenic genetic variants in familial and isolated keratoconus.
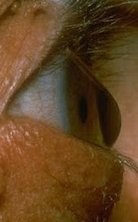
the cornea resulting from
keratoconus.
Additionally, we are studying the transcriptomes and proteomes of corneas in keratoconus subjects undergoing corneal transplantation to identify significant regulatory signaling networks in keratoconus. We identified increased expression of stress response genes, while expression of ECM genes as well as NRF2-regulated antioxidant and tissue-injury response related genes decreased in keratoconus (Shinde, 2019, Shinde, 2020). These findings are helping us to prioritize biologically significant candidate genes. Finally, we intend to use CRISPR-Cas9 gene editing approaches in cell and cornea organoid cultures to determine functional consequences of the variants we have identified. These studies will identify major genes, biomarkers and pathway that contribute to keratoconus that may be used to improve diagnoses and treatments for keratoconus.
Extracellular matrix interactions with immune cells and regulations of host response to infections and inflammation
We are interested in the role of small leucine-rich repeat proteoglycans (SLRPs), lumican, biglycan, and decorin in cell and tissue functions. As structural proteins of the ECM, these SLRPs interact with collagen fibrils and regulate collagen assembly and connective tissue structure. However, recent studies indicate additional interactions of SLRPs with cell surface receptors that influence cell migration, proliferation and induction of pro-inflammatory cytokines in an ECM that is remodeling during infections and inflammation. Our studies show that mice lacking lumican systemically, when challenged with bacterial infections of the cornea (Shao, 2013), or the lungs (Shao, 2012), are unable to clear the infection, and display more severe disease symptoms. We discovered that lumican released by activated fibroblasts, binds to the surface of neutrophils and promotes neutrophil migration (Lee, 2009). Lumican (Wu, 2007), and the other SLRPs interact with pathogen recognition receptors on macrophages and dendritic cells to modulate host response to infections. We have several projects studying how these SLRPs regulate bacterial and viral infections and autoimmunity.