
Basu Lab Research
Research projects led by investigators in the Basu Lab focus on two areas: understanding circuit interactions and single-neuron computations that help assign mnemonic valence to sensory signals; and determining the role of entorhinal cortex in modulating hippocampal plasticity and spatial representations.
Synaptic and Circuit Interactions between Hippocampus and Cortex to Shape Multisensory Processing
A critical step during sensory processing is the extraction of relevant information about the outside world from a host of distracting sensory inputs. One mechanism for generating salience is to associate sensory information from ongoing experiences with memories derived from past sensory experiences. Where and how these functional associations occur in the brain are central questions in neuroscience. This proposal aims to fill this gap by exploring circuit interactions and single neuron computations that help assign mnemonic valence to sensory signals.
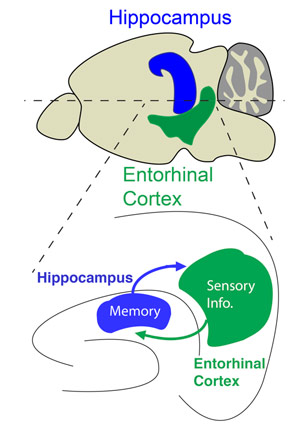
In this study, we propose that the hippocampus—the center of learning and memory—plays a crucial role in gating sensory information flow through its reciprocal circuit interactions with the entorhinal cortex, a hub for processing multisensory information. To test this hypothesis, we use anatomical and functional connectivity mapping experiments to validate how hippocampus communicates with entorhinal cortex output layers. We assess how hippocampal inputs modulate the short-term plasticity dynamics of excitatory-inhibitory synaptic transmission in the entorhinal cortex. Finally, we test whether the hippocampus actively modulates the synaptic strength and gain of sensory inputs to entorhinal cortex through dendritic integration and long-term plasticity mechanisms and how silencing the CA1 inputs to the entorhinal cortex affects contextual learning behavior.
Despite 60 years of research on memory processing, we know surprisingly little about the organization and function of hippocampal projection circuitry and the mechanisms by which memories modulate ongoing sensory processing in the entorhinal cortex. Our study will combine state-of-the-art in vitro and in vivo approaches, including electrophysiology, behavioral testing, and optogenetics, to provide a functional model of the unexplored hippocampal-entorhinal cortex reciprocal circuit.
In our new circuit model, the hippocampus directly transmits memory input to the entorhinal cortex to refine sensory output based on relevance and to quickly adapt behavior in response to changing environmental demands. Such a function could be used by the brain to facilitate reinforced learning, refine old memories, and form new memory associations. By identifying the neural circuit interactions between the hippocampus and entorhinal cortex, our study aims to improve our understanding of the mechanisms that underlie the memory-related sensory processing deficits experienced by patients of several neurological and neuropsychiatric illnesses including Alzheimer’s disease, schizophrenia, and PTSD.
Linking Plasticity of Hippocampal Representation Across the Single Neuron and Circuit Levels
Functional interactions between the entorhinal cortex and hippocampus are critical for spatial navigation and episodic memories related to people, places, objects, and events. Canonically, medial entorhinal cortex (MEC) processes spatial information while lateral entorhinal cortex (LEC) processes non-spatial contextual information. This ‘where’ and ‘what’ information is then projected to the hippocampus for formation of long-term representations associating the sensory and spatial features of the environment. Flexibility in hippocampal representations is critical for generating adaptive learnt behaviors and relies on plasticity.
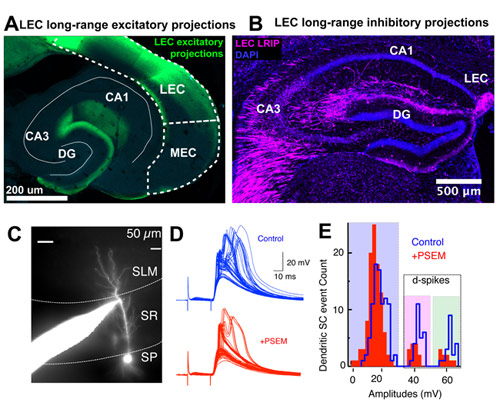
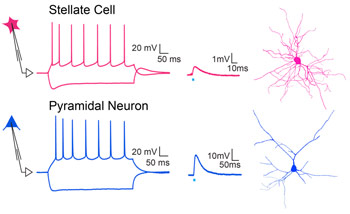
We propose a new role for the entorhinal cortex in modulating hippocampal plasticity and spatial representations. To test this, we dissociate the lesser-known organization and function of long-range and local circuit dialogue between LEC versus MEC and area CA3 of hippocampus during spatial coding.
This study tests the hypothesis that beyond the classically biased role of LEC inputs in non-spatial coding, coordinated activity of glutamatergic and newly discovered GABAergic input (Basu et al., 2016, Science) from both LEC and MEC is necessary for context-dependent plasticity of hippocampal place cells via gating of local excitation-inhibition dynamics and dendritic integration.
To test this idea, we have established innovative set of tools on the experimental and computational fronts to examine place cell plasticity across multiple levels. We perform intracellular electrophysiology from soma and dendrites of CA3 neurons in acute slices to functional map the LEC-CA3 circuit and read out CA3 place cell behavior at sub-cellular resolution with in vivo two-photon imaging of CA3 soma and dendrites as well as LEC axons in behaving animals during a head-fixed context morphing spatial navigational task.
Collaborations
In collaboration with Claudia Clopath, PhD, from Imperial College London, we are combining our experimental data with their computational modeling to build an integrated circuit centric model of plasticity in the hippocampus across multiple levels.
In collaboration with Cliff Kentros, PhD, from Kavli Institute of Systems Neuroscience at Norwegian University of Science and Technology, we are developing LEC cell type-specific mouse lines for multiplexed optogenetic activation and silencing of glutamatergic and GABAergic inputs simultaneously or alternatingly and read-out how these manipulations impact CA3 plasticity. We are building a unique computational model of hippocampal place cell coding at single neuron and network levels incorporating modulation of dendritic excitation-inhibition and long-term plasticity. NYU Langone researchers György Buzsáki, MD, PhD, and Dmitry Chklovskii, PhD, provide expert consultation on place cell and large-scale imaging data analysis.
Our study provides a unique perspective on long-range and local circuit dynamics that impart flexibility to otherwise stable neuronal representations of space based on environmental demands.