Rodriguez Lab
Investigators in NYU Langone’s Rodriguez Lab focus on two parasites: Plasmodium, which causes malaria, and Trypanosoma cruzi, which causes Chagas disease.
Malaria is a devastating disease that causes more than 400,000 deaths per year, mainly among African children. Despite many efforts to control the disease with anti-malarial drugs and insecticides to eliminate mosquito vectors, the appearance of resistant populations of parasites and mosquitoes respectively have impaired the efficacy of these approaches. There is an urgent need for new strategies to control malaria, but there is a lack of detailed knowledge of the basic biological processes of Plasmodium that would allow faster development of anti-malaria drugs and vaccines.
A main interest of our laboratory is the study of malaria-induced inflammatory pathology. We have found that Plasmodium synergizes with oxidative stress to activate the inflammasome and the production of cytokines. This novel pathway that regulates anti-malaria inflammatory response opens the possibility of new exciting approaches to control disease pathology and death.
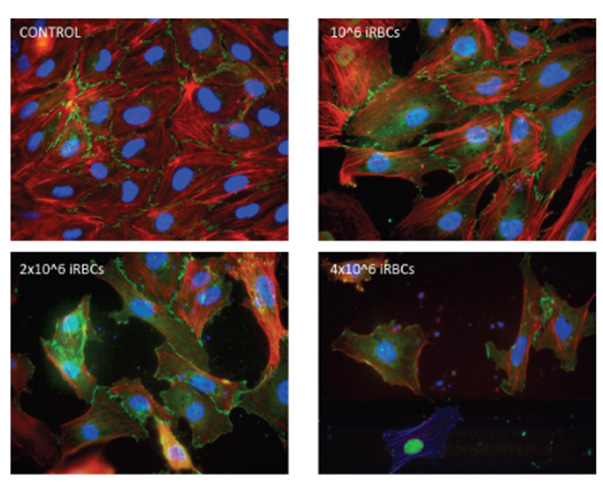
We also study cerebral malaria, a complication of severe malaria that frequently leads to coma and death. During cerebral malaria, erythrocytes infected with Plasmodium falciparum bind to endothelial cells in the brain blocking normal circulation. We have observed that infected erythrocytes induce the rupture of inter-endothelial cell junctions and detachment of endothelial cells. Our work is focused on the inhibition of this process, with the aim to find inhibitors that would preserve the integrity of the endothelium during cerebral malaria and would allow the development of a specific treatment against cerebral malaria.
Malaria-induced anemia is another important cause of death by this disease. We have observed that autoimmune antibodies that are induced by malaria contribute to the loss of uninfected erythrocytes. In particular, autoantibodies that recognize phosphatidylserine (PS), a lipid normally found in the inner leaflet of the plasma membrane, are generated during malaria in mice and in human patients. During malaria, uninfected erythrocytes expose PS and anti-PS antibodies bind to them facilitating their clearance. We have identified FcRL5+ T-bet+ B cells, which are characterized by their involvement in autoimmune diseases, as main producers of these anti-PS antibodies. Patients with malaria have elevated levels of these cells, which correlate with anti-PS antibody levels and with anemia. Our hypothesis is that anti-PS antibodies bind to uninfected erythrocytes exposing PS inducing their clearance during malaria.
In addition to our focus on malaria, we collaborate with a pharmaceutical company, GlaxoSmithKline (GSK), to develop effective drugs against Chagas disease. In collaboration with GSK, we have performed a high throughput screening of two million compounds for intracellular Trypanosoma cruzi to find compounds with anti-trypanosomal activities. Selected compounds are being tested for efficacy in mice.
Contact Us
Ana M. Rodriguez, PhD
Principal Investigator
Professor, Department of Microbiology
Director, Anti-Infectives Screening Core
Co-Director, Anopheles Insectary
Alexandria Center for Life Science – West Tower
430 East 29th Street, AW 3rd Floor
Room 511 (Office), Room 524 (Lab)
New York, NY 10016
Office Phone: 646-501-6997
Lab Phone: 646-501-6996
Email: ana.rodriguez@nyulangone.org