
Dopamine Effect on Motor Learning & Aberrant Plasticity in Parkinson’s Disease
The mechanism of the widely-prescribed drug, levodopa, and other dopaminergic drugs in treating motor impairment in Parkinson’s disease (PD) is known mostly for its benefit in motor performance and clinical development is focused on alleviating the progressive shortening in the time for which each dose of levodopa is effective.
We study the lesser known, but more critical component, of the therapeutic responses called the long-duration response (LDR). Each dose of levodopa has a long-lasting therapeutic effect that decays gradually upon levodopa discontinuation. We developed the first animal model of LDR and proposed the mechanism to be motor learning mediated by dopamine effect on striatal plasticity. We found that this progressive decay is only seen with continued performance of the same task, and that it activates the “no-go” pathway of the basal ganglia—a brain region important for movement—whose function is severely impaired in Parkinson’s disease.
Using a novel technique to visualize and manipulate subpopulations of neurons activated by specific motor tasks, we will examine whether the decay of levodopa’s long-lasting therapeutic effect is caused by the pathological activation of an “incorrect” subpopulation of “no-go” pathway neurons. Such confirmation may lead to development of entirely novel treatment approaches that reverse motor impairment in Parkinson’s disease.
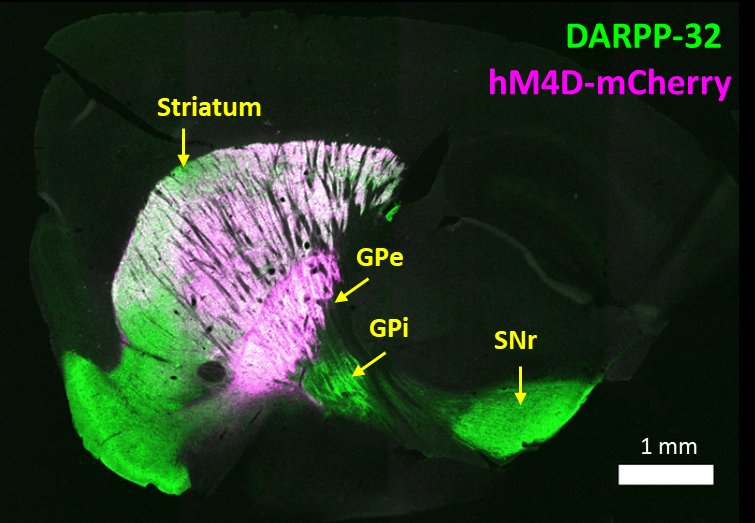
Cheung et al. Learning critically drives parkinsonian motor deficits through imbalanced striatal pathway recruitment. PNAS. 2023. DOI.