Modulation of Basal Ganglia Output from the SNr in Parkinson’s Disease & Levodopa-Induced Dyskinesia
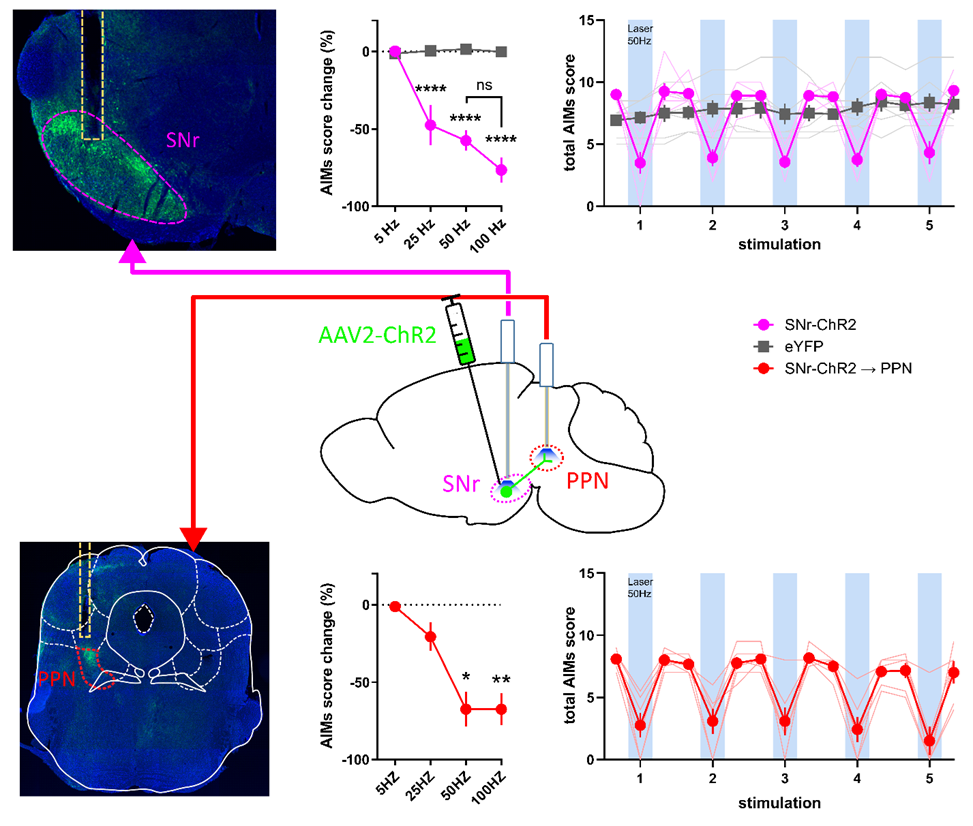
Long-term use of levodopa for Parkinson's disease (PD) treatment is often hindered by development of motor complications including levodopa-induced dyskinesia (LID). The substantia nigra pars reticulata (SNr) and globus pallidus internal segment (GPi) are the output nuclei of the basal ganglia. Dysregulation of SNr and GPi activity contributes to PD pathophysiology and LID. In NYU Langone’s Kang Lab, we study whether direct modulation of SNr GABAergic neurons and SNr projections to the pedunculopontine nucleus (PPN) regulates PD symptoms and LID in a mouse model. We express Cre-recombinase activated channelrhodopsin-2 (ChR2) or halorhodopsin (NpHR) AAV2 vectors selectively in SNr GABAergic neurons of Vgat-IRES-Cre mice in a 6-hydroxydopamine model of PD to investigate whether direct optogenetic modulation of SNr neurons or their projections to the PPN regulates PD symptoms and LID expression. The forepaw stepping task, mouse LID rating scale, and open field locomotion are used to assess akinesia and LID, respectively, to test the effect of SNr modulation.
Akinesia is improved by suppressing SNr neuron activity with NpHR. LID is significantly reduced by increasing SNr neuronal activity with ChR2, which does not interfere with the anti-akinetic effect of levodopa. Optical stimulation of ChR2 in SNr projections to the PPN recapitulates direct SNr stimulation. Modulation of SNr GABAergic neurons alters akinesia and LID expression in a manner consistent with the rate model of basal ganglia circuitry. Moreover, the projections from SNr to PPN likely mediate the antidyskinetic effect of increasing SNr neuronal activity, identifying a potential novel role for the PPN in LID. Further studies are examining the role of PPN subregions and specific cell types in mediating these effects.