
Morgan Lab Research
Investigators in the Morgan Lab study the genetic and epigenetic basis for how premalignant phases of disease transition to myeloma and beyond.
Multiple Myeloma
Multiple myeloma is a disease that results from malignant transformation of normal plasma cells that have developed the "hallmarks of cancer” as a result of acquired genetic changes. The clinical transformation system of monoclonal gammopathy of undetermined significance (MGUS) through smoldering multiple myeloma (SMM) to multiple myeloma provides an excellent model to understand how disease drivers lead to cancer progression. In essence, we use this system to understand both the molecular and cellular basis for the transition of a normal plasma cell to one with the hallmarks of cancer (see Figure 1).
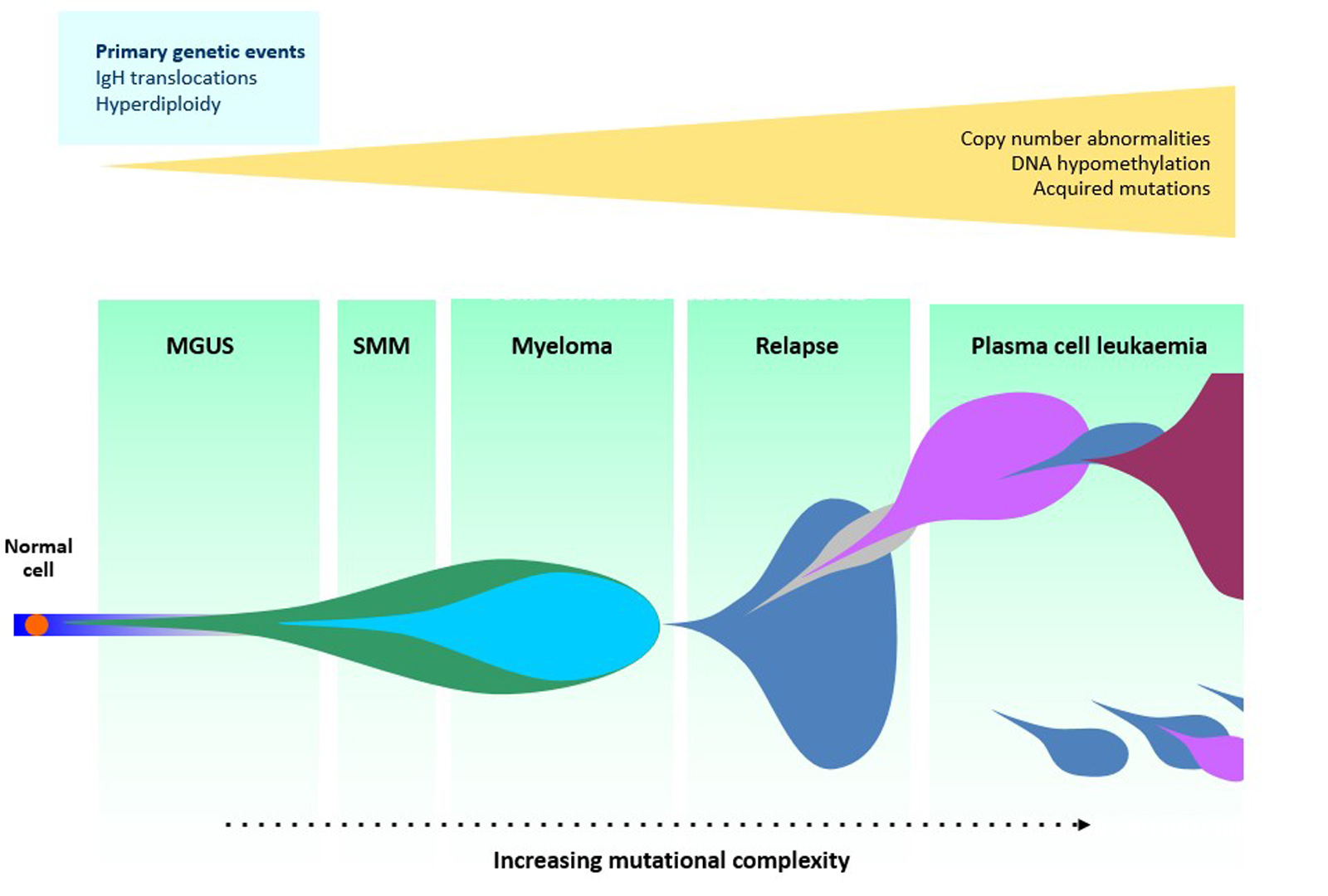
Our current concept of multiple myeloma development is based on the immortalization of a normal plasma cell, which is then subject to Darwinian evolution in the bone marrow. This “myeloma initiating cell” progresses to multiple myeloma by the acquisition of genetic events that confer upon it a selective growth advantage within the bone marrow.
At a molecular level the “clonal initiating cell” is immortalized by a mutational event generated by an “off-target” effect of the normal germinal center reaction important in the differentiation of normal B-cells into plasma cells during an immune response.
These initiating events are either an abnormal structural rearrangement, where the immunoglobulin gene locus is placed next to a critical oncogene leading to its overexpression, or are gains of the odd-numbered chromosome, hyperdiploidy.
Once established, the immortalized “myeloma-initiating cell” acquires additional driver genes that deliver the growth advantage within the bone marrow “plasma cell niche” leading to clonal selection. The end result of this process is cancer progression by selective sweeps of the best adapted clones.
Late in the evolutionary process, if the clonal cells develop the ability to grow independently of the bone marrow plasma cell niche, they may proliferate in the blood as plasma cell leukemia.
Waldenstrom’s Macroglobulinemia
Waldenstrom’s macroglobulinemia is part of the group of lymphoplasmacytic lymphomas characterized by small B-cells at various stages of plasmacytic differentiation encompassing a wide range of B-cell differentiation states in each case.
Immunoglobulin gene sequence analysis shows that tumor cells are related to IgM memory B-cells. However, other immunophenotypic and morphologic features suggest a relationship to plasma cells, which makes the assignment of the stage of origin challenging. However, Waldenstrom’s macroglobulinemia has recently been separated into two groups based on its pattern of DNA methylation, one with memory B-cell pattern together with a second group with a more plasma cell phenotype.
Despite the phenotypic differences that have been identified, there is monotypic light chain usage and clonal IGHV rearrangements between lymphocytic and plasmacytic populations, consistent with a common clonal origin. Different molecular features are seen in each of these two groups suggesting that the differentiation is associated with specific molecular variants.
AL Amyloid
Amyloid refers to the tissue deposition of insoluble protein that is bi-refrigent on Congo red staining. We are predominantly in the clinic treatment of this disorder and how it relates to the pre-malignant MGUS phase of multiple myeloma.