
Treisman Lab Research
Research in the Treisman Lab seeks to understand how cells in the developing visual system communicate with each other to take on specific fates, build functional visual structures, and connect up into neural circuits.
Cell Fate Determination
The eye is made up of photoreceptor neurons as well as several types of non-neuronal support cells that produce the corneal lens and screening pigments. All these cells arise from a common pool of progenitors, raising the intriguing question of how their distinct fates are specified.
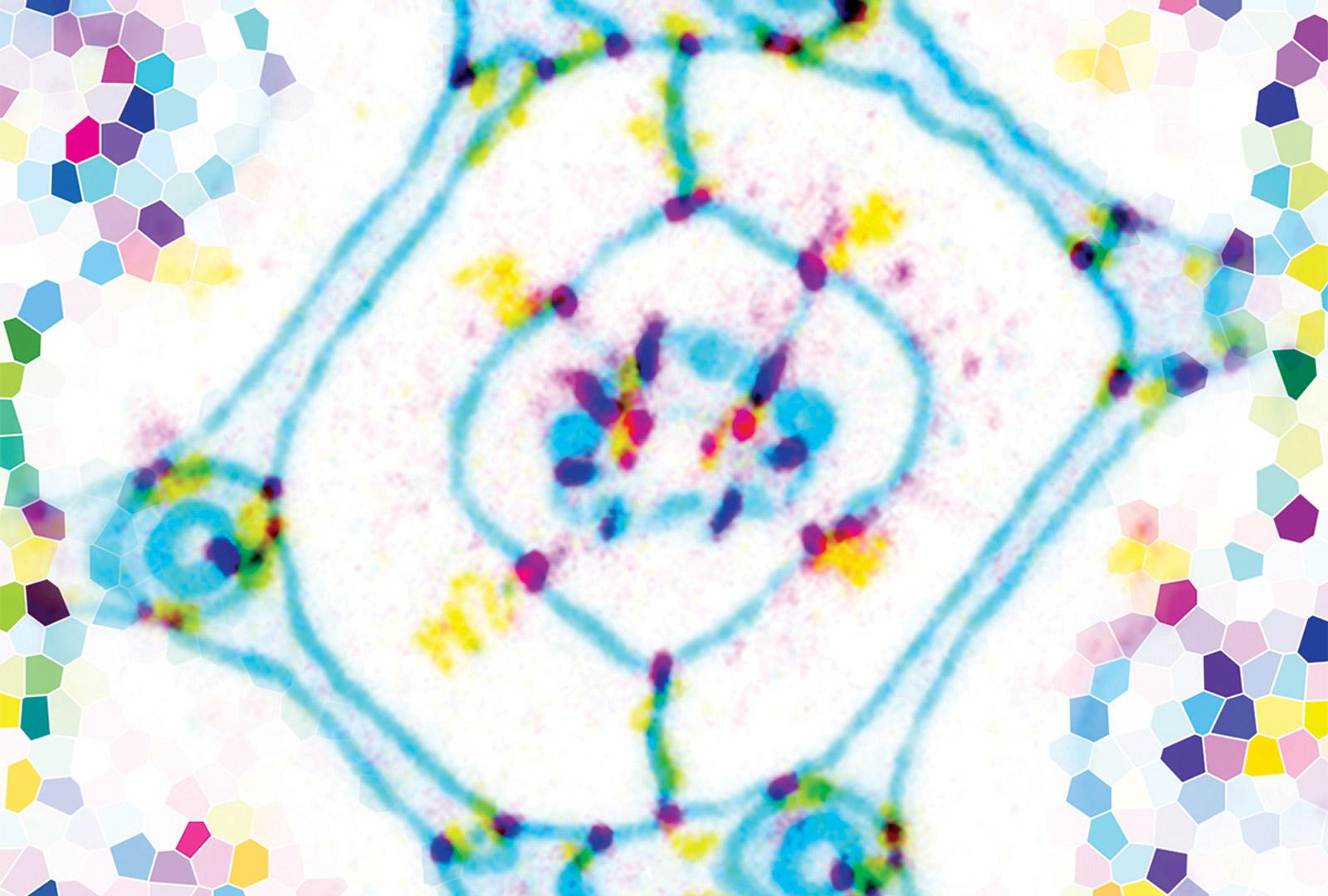
We would like to understand how cell-cell signaling interacts with intrinsic transcription factors to induce cells to take on specific fates in a characteristic sequence. The Glass transcription factor was previously thought to determine the photoreceptor identity. However, we have found that it promotes the terminal differentiation not only of photoreceptors, but of all the cell types in the eye (Morrison et al., 2018). We are searching for other transcription factors that enable Glass to activate different target genes in different cellular contexts.
Epidermal growth factor receptor (EGFR) signaling is also important for the differentiation of most eye cell types, and can synergize with Glass to transform naïve cells towards eye-specific fates. We are using transcriptomics and transcription factor binding data to understand how Glass and the EGFR pathway interact at the molecular level to activate different gene expression programs (Wang et al., in preparation).
Cell Rearrangement
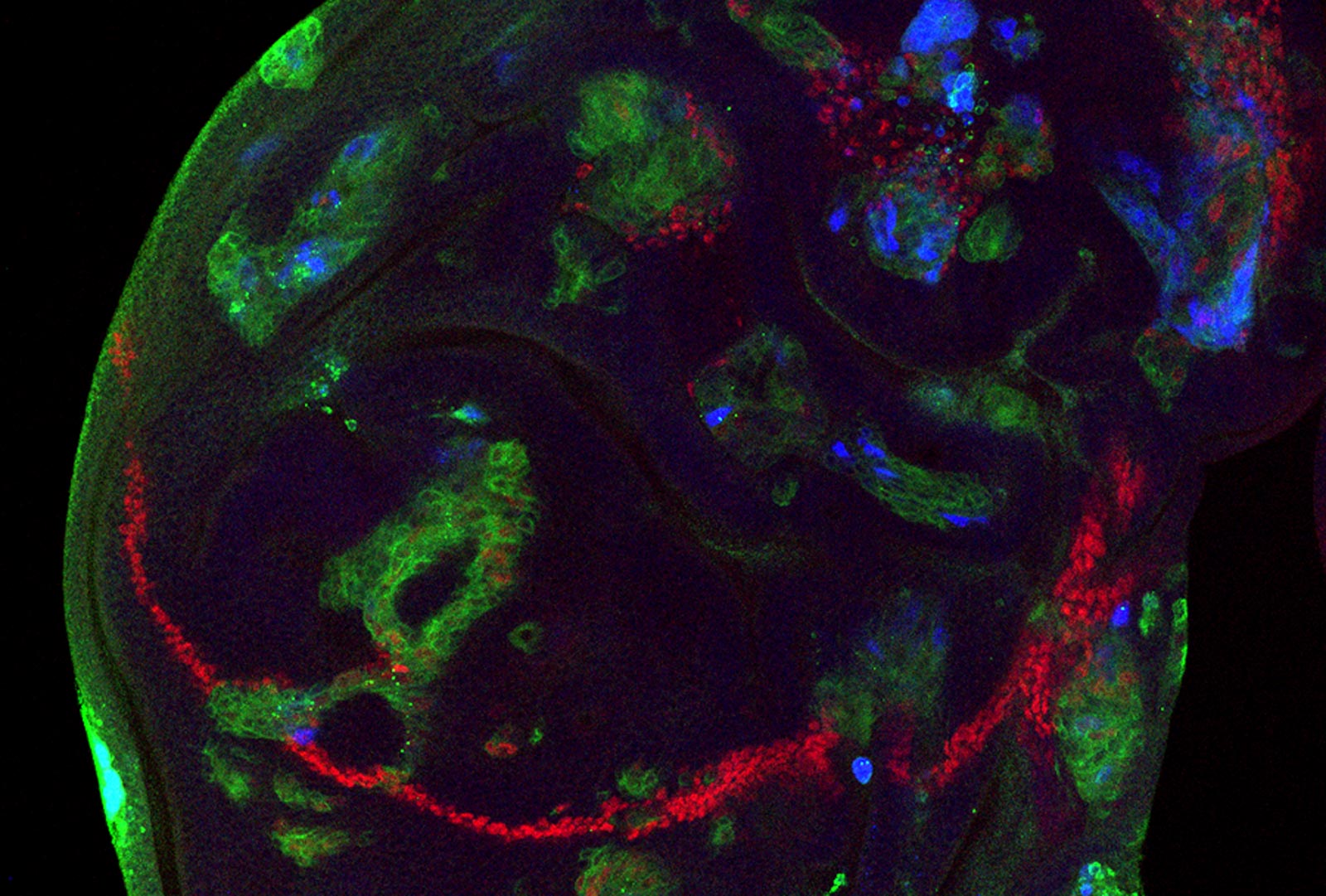
Following cell fate specification, retinal cells rearrange and change their shapes within the epithelium to produce a highly regular hexagonally packed lattice that enables accurate vision.
We recently showed that these cell rearrangements require the immunoglobulin family protein Sidekick (Sdk), which we identified as the first known component specific to tricellular adherens junctions. Sdk interacts with ZO-1 and Afadin homologues to tether actin filament ends and resist tension on these cell contacts during junction shortening (Letizia et al., 2019), and recruits the WAVE regulatory complex to promote actin branching during junction lengthening (Malin et al., 2022). We have found other proteins that interact with Sdk and that may explain how it is specifically localized to tricellular contacts and how it controls the growth and shrinkage of bicellular junctions.
Synapse Formation
Establishment of the correct neural circuitry in the visual system requires growing axons to recognize the appropriate cells as synaptic partners. The photoreceptors that mediate color vision provide us with a simple system in which to examine this process, as photoreceptors that respond to different wavelengths of light project to different target layers in the brain.
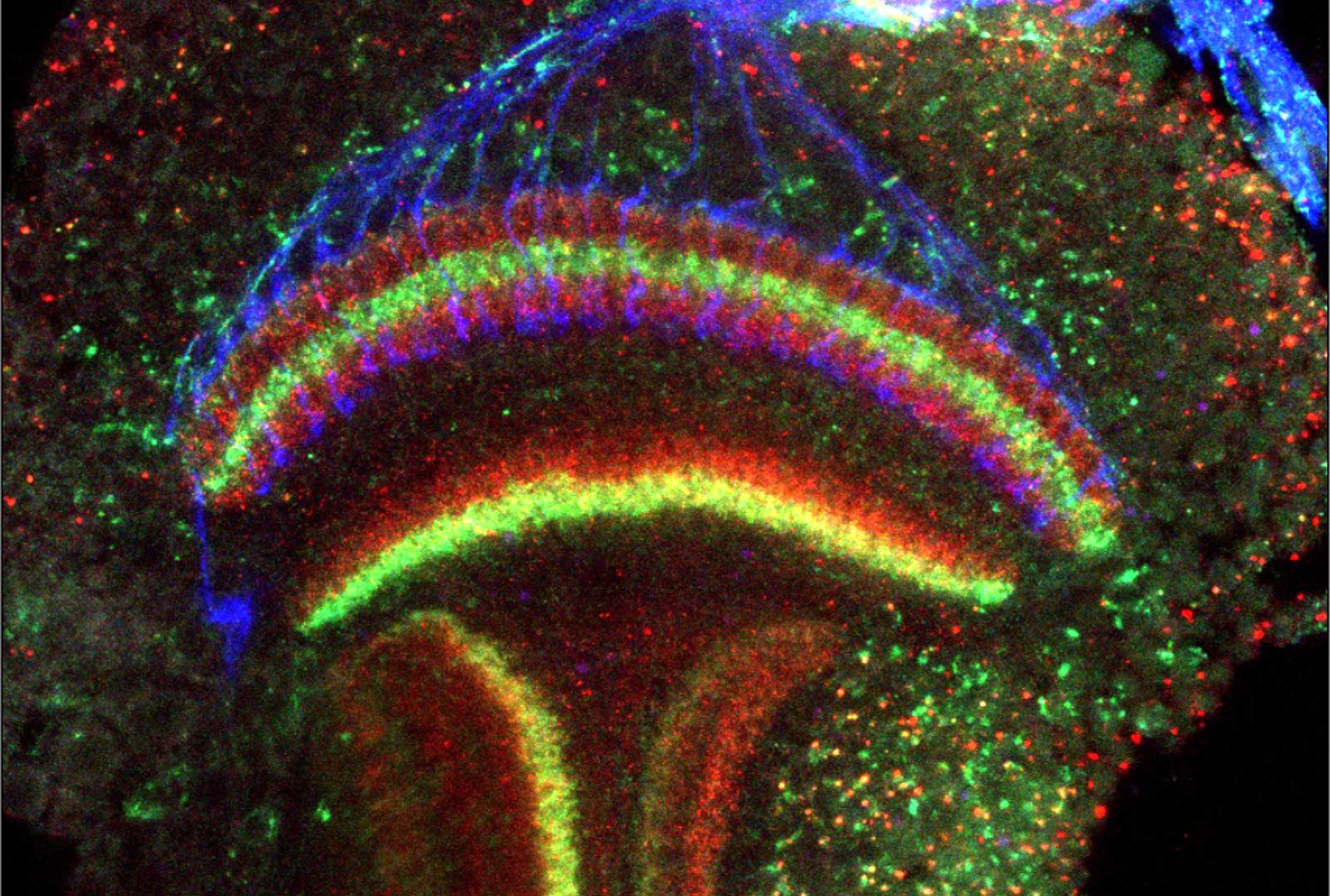
We have shown that ultraviolet-sensitive R7 photoreceptors require the receptor protein tyrosine phosphatase Lar and its adaptor protein Liprin-alpha to find the correct postsynaptic partners (Maurel-Zaffran et al., 2001; Hofmeyer et al., 2006, 2009). More recently, we found that a novel CUB-domain protein, Lost and found (Loaf), is required in R7 only if it is also present in neurons in the brain that form synapses with R7, suggesting that the pre- and postsynaptic cells must have matched Loaf levels to form stable connections (Douthit et al., 2021).
In searching for additional sources of guidance information that direct R7 axons to the correct layer, we have found that the axon guidance molecule Plexin A acts in neurons that grow tangentially across the R7 target region as a cue that establishes the overall layered structure of the region (Bustillo et al., in preparation).
Another interesting question is how synapses grow to reach the correct size during development. We are using the neuromuscular junction as a model to understand how synapse growth is coordinated with growth of the target muscles. We have found that Insulin receptor pathway signaling locally increases postsynaptic differentiation, which promotes the elaboration of presynaptic structures by motor neurons. A specific isoform of the RhoGEF dPix plays a central role in this signaling process (Ho and Treisman, 2020). We would like to know how Insulin signaling controls dPix activity and how motor neurons sense the extent of postsynaptic differentiation.
Curvature of the Corneal Lens
Like the mammalian cornea, the fly corneal lens is a curved structure made up mostly of extracellular matrix that focuses light onto the photoreceptors. The major components of the corneal lens are secreted by the cone and primary pigment cells underneath its center. However, we have found that loss of the transcription factor Blimp-1 specifically from the peripheral secondary and tertiary pigment cells makes the external surface of the lens flat (Wang et al., 2022).
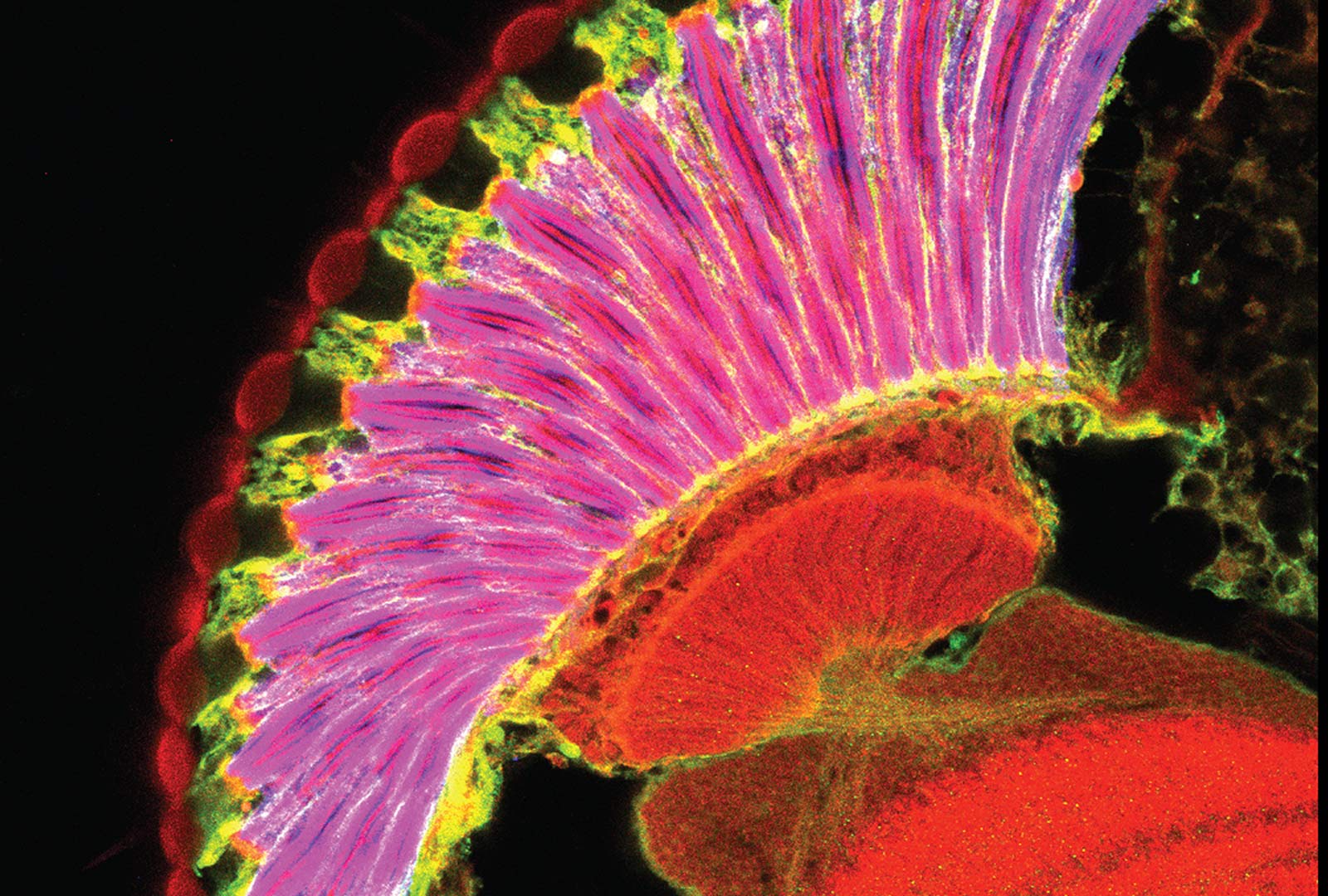
We are investigating which of the genes downstream of Blimp-1 contribute to this phenotype, and have found potential roles for Zona Pellucida domain proteins, lectins, and a gene that is frequently mutated in human corneal dystrophies.