
Molecular Basis of Neuropathological Behavior
Genetics tells us that damage to synaptic and nuclear proteins are at the root of major diseases of the brain. One such disorder, autism, has recently emerged as a major interest of our laboratory. A rare form of autism known as Timothy Syndrome (TS) stems from a single amino acid change in an L-type channel known as CaV1.2. So far, we have focused on characterizing the behavior of the channel itself, and of mice bearing the mutation. Remarkably, those animals show significant behavioral alterations reminiscent of autism, including persistent behavior, altered communication, and reduced social interaction relative to their unaffected littermates.
Current efforts in the Tsien Lab center on how the autistic features are generated and how they might be counteracted pharmacologically. Interestingly, the same gene for CaV1.2 has also been implicated in major depressive disorder and in schizophrenia. The alluring possibility is that genetic clues about those diseases can help guide our basic research, and that information will someday flow in reverse. Like many others, we relish the challenge of integrating pieces of neuroscience, genetics, and cell biology.
Related Publication
Bader PL … Shamloo M. Mouse model of Timothy syndrome recapitulates triad of autistic traits. PNAS. 2011. DOI.
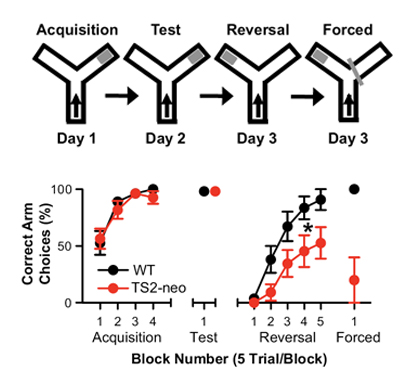
Autism spectrum disorder (ASD) typically arises from a mixture of environmental and genetic factors. However, in some rare cases like Timothy syndrome (TS), a specific, single gene mutation is sufficient to generate autism. A mutation in the gene for Cav1.2 L-type calcium channel is present in both TS1 and TS2. We generated viable heterozygus TS2-neo mice, and characterized the behavioral phenotype as a model of ASD in mice. TS2-neo mice showed repetitive and perseverative behavior, altered social behavior, altered vocalizations and enhanced tone- and context-cued fear conditioning.