
IGF1 Receptor
The IGF1 receptor is highly related in sequence and structure to the insulin receptor, but has distinct biological functions, one of which is cell survival. Therefore, this RTK is a potential target for inhibition in tumor cells.
In collaboration with Dr. Todd Miller at SUNY Stony Brook, researchers in the Hubbard Lab have determined the three-dimensional structure of the IGF1 receptor kinase domain using X-ray crystallography (see figure 1). Several amino acid differences between the IGF1 receptor and the insulin receptor near the ATP binding cleft might be exploited by small-molecule inhibitors to gain selectivity for the IGF1 receptor over the insulin receptor. To this end, structural studies of inhibitors bound to the IGF1 receptor kinase are being pursued.
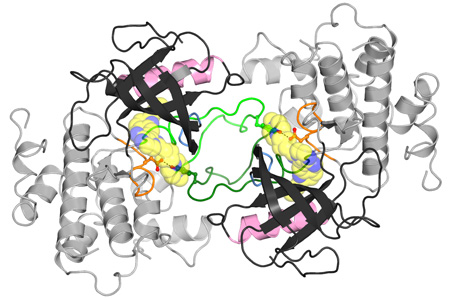
Read more in our paper “Small-molecule inhibition and activation-loop trans-phosphorylation of the IGF1 receptor” published in The EMBO Journal.