
MuSK
Another RTK of interest is MuSK, which is expressed exclusively in muscle cells and plays an essential role in the formation of neuromuscular synapses by promoting clustering of acetylcholine receptors. Activation of MuSK by agrin results in autophosphorylation of several tyrosines in the cytoplasmic domain of MuSK.
In a collaboration with Steven J. Burden, PhD, researchers in the Hubbard Lab have determined the crystal structure of the cytoplasmic (tyrosine kinase-containing) domain of MuSK to understand how kinase activity is regulated in this receptor. The structure reveals that MuSK is strongly autoinhibited by the kinase activation loop (see figure 1).
Read more in our paper “Crystal structure of the agrin-responsive immunoglobulin-like domains 1 and 2 of the receptor tyrosine kinase MuSK” published in Journal of Molecular Biology.
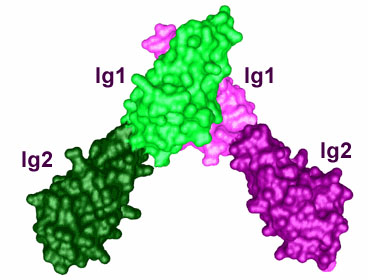
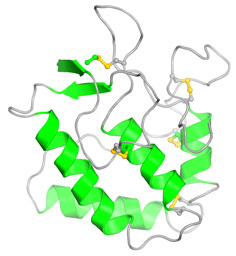
In addition to determining the crystal structure of the tyrosine kinase domain of MuSK, we have determined crystal structures of the first two immunoglobulin-like domains (Ig1-2) and the Frizzled-like cysteine-rich domain (Fz-CRD) of the MuSK extracellular region (see figure 2). Ig1-2 crystallized as a dimer, mediated by Ig1, and the residues in the dimer interface are critical for agrin-induced phosphorylation of the receptor. Whether these residues are important for receptor dimerization or for a heterologous interaction (for example, with the agrin receptor LRP4) is still under investigation.
Read more in our paper “Crystal structure of the frizzled-like cysteine-rich domain of the receptor tyrosine kinase MuSK” published in Journal of Molecular Biology.
Dok7
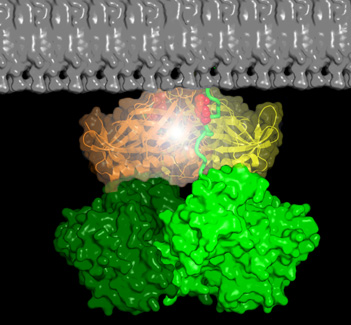
Dok7 is a cytoplasmic adaptor protein that contains tandem PH and PTB domains in the N-terminal portion of the molecule. In muscle, Dok7 is required for MuSK activation, and the structure of the PH and PTB domains revealed that these domains form a dimer that binds to the juxtamembrane phosphotyrosine (pY553) in MuSK, facilitating phosphorylation of the kinase activation loop (see Figure 3).
Read more in our paper “The cytoplasmic adaptor protein Dok7 activates the receptor tyrosine kinase MuSK via dimerization” published in Molecular Cell.