
B-cell Epitopes in Staphylococcus Aureus
Staphylococcus aureus (Sa) is a significant human pathogen in both hospital and community settings. Sa-related illness affects approximately 1.3 million individuals, resulting in more than 300,000 hospitalizations and more than 40,000 deaths in the United States annually. In fact, in 2005 more people died of Sa infections than from HIV infection and AIDS. The majority of Sa infections are caused by highly virulent drug-resistant strains. A substantial number are community-acquired strains and occur in otherwise healthy individuals. The remaining cases are hospital-acquired infections, of which there has been little decline in total incidence since the emergence of multi-drug resistant Sa (such as MRSA) in the early 1990s.
Given the large number of individuals who contract MRSA infections annually, many are refractory to traditional courses of antibiotic treatment. To date, no approved vaccine is available. Thus, the development of a vaccine and/or protein-based treatment modalities against virulence factors critical for the infection process are attractive therapeutic options.
In the B-cell Immunobiology Lab, we have built a program that coordinates collaborations with three other NYU Langone investigators: Victor J. Torres, PhD, in the Department of Microbiology; Bo Shopsin, MD, PhD, in the Department of Medicine’s Division of Infectious Diseases; and Beatrix M. Ueberheide, PhD, in the Department of Biochemistry and Molecular Pharmacology. We are currently recruiting subjects with soft tissue and skin infections. From each infection, the causative Sa clone as well as PBMC and serum/plasma samples are biobanked for later analysis. These studies are dedicated to the identification and validation of B-cell epitopes important for immune defenses against this re-emerging pathogen.
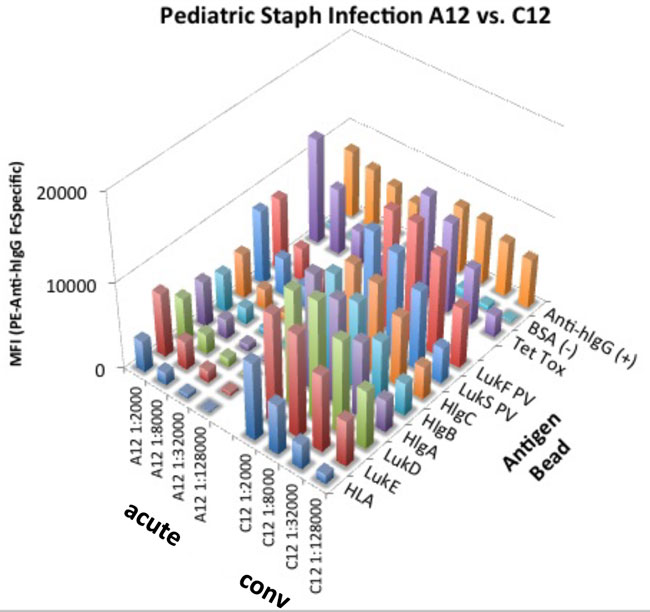
With our current bead-based multiplex MagPix assay, we have evaluated immune responses detected in clinical samples from our Bellevue-NYU cohort, during acute and convalescent phases of S. aureus (MSSA and MRSA) infection. We have studied seven such paired longitudinal serum samples from patients who recovered from MRSA and MSSA infection, with an average of 42 days follow-up (range 36-49 days). We assayed IgG reactivity in samples diluted 100, 1000, 10,000, and 100,000-fold in PBS/1% BSA Fraction V/0.05% Tween-20, and applied 1000 beads per antigen per well. We then applied our standard assay procedure for magnetic microsphere-based detection of IgG responses using an anti-human IgG (Fab) Fc-region-specific PE conjugate diluted 1:250 in PBS/1%BSA Fraction V/0.05% Tween-20. The results showed that, generally, only very minor changes were seen in IgG reactivity profiles for specific S. aureus antigens between visit 1 (acute) and visit 2 (convalescent). Recombinant antigens for these studies were provided by Dr. Torres. Studies performed by Adam Pelzek.
Our work is supported by a contract from the National Institute of Allergy and Infectious Disease: NIAID-HHSN272201400019C, “B Cell Epitope Discovery and Mechanisms of Antibody Protection.”