
Regulatory Antibodies
In recent studies, researchers in the B-cell Immunobiology Lab have defined the in vitro and in vivo properties of a class of naturally arising regulatory antibodies, which discriminate apoptotic cells from healthy cells. We have shown that with the optimal antibody-mediated effector functions, these antibodies have homeostatic properties that enhance the phagocytic clearance of dead and dying cells, and blunt inflammatory responses to agonists to a range of TLR. These antibodies also oppose IgG immune complex mediated inflammatory responses. We have shown that these activities provide therapeutic benefits in two models of inflammatory arthritis and prolong survival in a murine lupus model. Most importantly, from the characterization of the functional properties in IgM anti-ACM antibodies that correlate with clinical benefits, we have developed bench marking assays and validated the design of recombinant murine IgG anti-ACM antibodies.
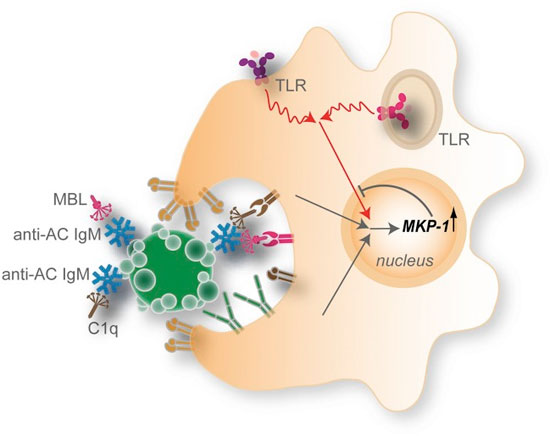
IgM antibodies to determinants on ACs recognize and form complexes with ACs (or AC microparticles), which recruit high levels of C1q and MBL. This AC–immune complex forms an interaction with DCs that may involve integrins, receptors for C1q and MBL, and other AC receptors. In quiescent DCs, these complexes may result in little or no effects on MAPK signaling. However, in the context of stimulation by agonists for endosomal (TLR3, -7, and -9) or membrane-associated (TLR4) innate immune receptors, there is an integration of signals with those from NAb–MBL/C1q–AC complexes that result in high nuclear expression of the anti-inflammatory phosphatase MKP-1. This pathway contributes to inhibition of the activation and nuclear localization of the primary MAP kinases and their downstream proinflammatory substrates. (See Grönwall, et al., 2012, PNAS.)
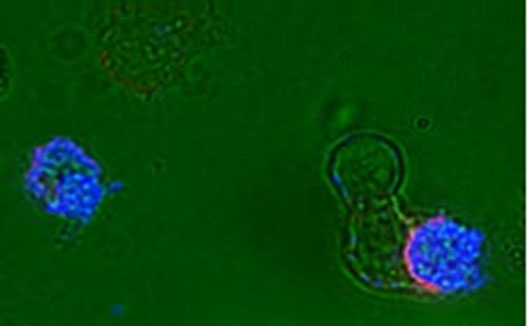
Dendritic cells dying by apoptosis undergo characteristic nuclear condensation, and are specifically recognized by a natural antibody (IgM anti-phosphoryl choline) to apoptotic cell membrane determinants (blue), and such an apoptotic cell is seen to be ingested at lower right by an immature dendritic cell via a phagocytic cup lined with the CD11c (CR4)(red). (See Chen et al., 2009, Journal of Immunology.)
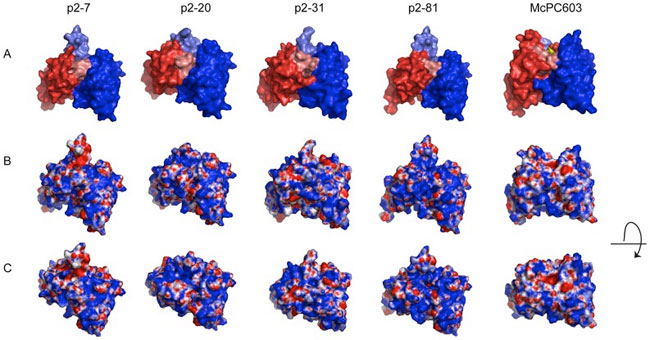
The above models are compared to the crystal structure of the PC-binding murine antibody McPC603, with the PC-antigen in the binding pocket (PDB ID: 2MCP). A) The VL region is visualized in red and the VH region in blue. HCDR3 is highlighted in lighter blue shade and LCDR3 in lighter red shade. B) Electrostatic surface models of the variable regions. Blue color represents positively charged surface residues and red negatively charged residues. C) Electrostatic surface models with a view looking into the potential antigen-binding site. Models of the variable region surfaces were generated by WAM tool, using dead-end elimination for the side-chains building and VFF energy screen method. Illustrations of the structures were generated in PyMol (DeLano Scientific LLC) using APBS for electrostatic calculations. (See Grönwall et al., 2014, PLoS One.)