
Longitudinal Microbiome Analyses & Lupus Disease Flares
While the study of the gut microbiome is a young field, it has long been known that bacterial exposures can trigger autoimmune conditions. In particular, for more than a century, bacterial infections that breach the skin and mucosal barriers have been implicated as common triggers of autoimmune syndromes, especially post-infection autoimmune diseases that include rheumatic fever and post-streptococcal glomerulonephritis.
Only in the past few years has the importance of imbalances within our commensal microbiota communities, and especially those that reside within the gut, been appreciated as contributors to autoimmune disease. These contributions may be important even when these microbes do not themselves cause infection. Several microbial species and a range of disease mechanisms have been implicated.
Bacteria that are part of natural bacterial resident communities are called symbionts, but when such a bacterial species at times causes disease (or pathology), these are called pathobionts. Emerging data suggest that expansions (or blooms) of pathobiont species are involved in autoimmune pathogenesis and stimulate clonal expansion of T cells and B cells that recognize microbial antigens.
From studies of families with multiple members that have an autoimmune disease, it is well documented that even though many individuals have genetic susceptibility for systemic lupus erythematosus (SLE), not everyone with inherited risk develops the disease. However, in most cases, the triggers for clinical lupus disease flares have remained elusive.
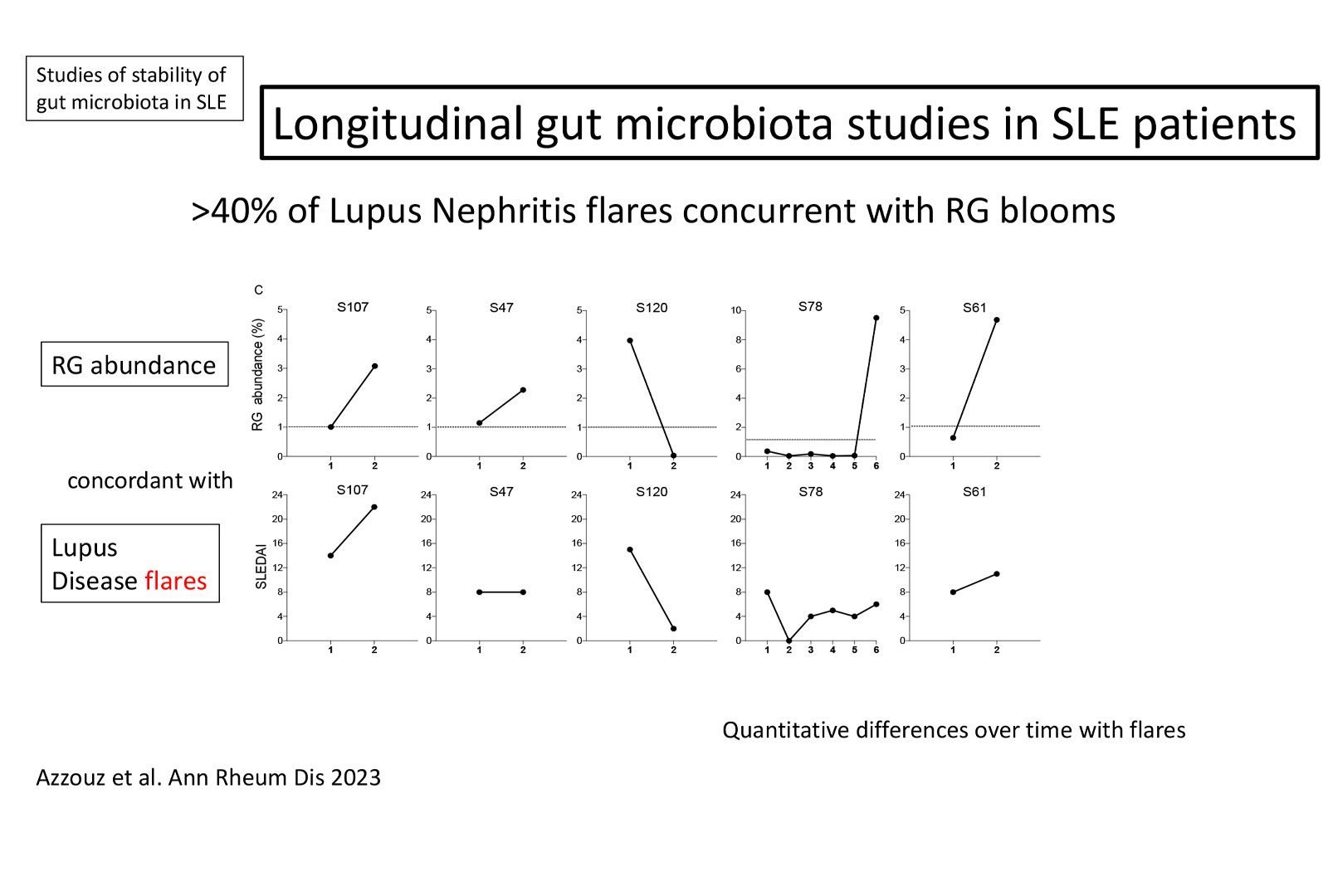
In the B-cell Immunobiology Lab, integral to our investigations of the relationships between microbiota community resilience and disease activity, we have reported the first longitudinal analyses of gut-microbiota communities in individual SLE patients. Of note, expansions of the anaerobic commensal pathobiont, Ruminococcus (blautia) gnavus (RG) only occurred at times of high-disease activity, and this expansion were detected in almost half of the patients during lupus nephritis (LN) disease flares. Our laboratory is continuing to explore these findings, as we postulate that blooms of the RG pathobiont are common drivers of clinical flares of often remitting-relapsing lupus disease.
A key finding is that disruption of the gut barrier can result in the release of gut bacterial antigen into the body. We are therefore investigating how increased intestinal permeability, which reflects functional impairment of the gut barrier, allows microbial products and microbial metabolites to interact with host immune cells, subsequently driving progression of autoimmune pathology.